AML arising from HMA-treated myeloid disorders, also known as “treated-secondary AML” (ts-AML) is associated with an adverse prognosis (Complete Remission [CR] rates 15-30% and median Overall Survival [OS] 6-10 months). E-selectin, a marker of cell survival and resistance to chemotherapy, is highly expressed in the leukemic microenvironment. Exposure of leukemic blasts to HMAs has been shown to increase its expression. Uproleselan is an e-selectin antagonist that has demonstrated antileukemic activity in AML. We sought to study the combination of low-intensity chemotherapy with Cladribine + LDAC (CLAD/LDAC) with uproleselan to overcome local and microenvironmental resistance.
This Phase Ib/II clinical trial (NCT04848974) evaluate the safety, tolerability, and efficacy of Uproleselan added to Cladribine and LDAC. A 3+3 dose-escalation approach was implemented to evaluate 2 different dose levels for Cladribine (CLAD)+ LDAC; each 4-week cycle consists of Uproleselan (at fixed dose of 800mg intravenously [IV]). Induction and reinduction if required, included IV uproleselan twice a day from day 1-12; consolidation was given same days but once a day if patients(pts) achieved response (complete response[CR] with incomplete hematological recovery [CRi] or morphologic leukemia-free state [MLFS]). CLAD was IV for 5 days (3.75mg/m2 and 5mg/m2; level -1 and 1 respectively) and subcutaneous LDAC twice daily 10 days (15mg, and 20mg; level -1 and 1 respectively) during induction; consolidation was similar except it was with 3-days of CLAD, for up to 6 cycles, pts aged ≥18 years with a diagnosis of ts-AML with adequate organ function, who have not received therapy for their AML were enrolled. ts-AML is defined as AML arising from previously treated myeloid neoplasm. Presence of the e-selectin was assessed as exploratory analysis.
20 pts have been treated, 18 pts evaluable: 14 (70%) male, median age was 72 yrs (range, 58-86); at the start of therapy, the median bone marrow blasts were 26.5% (20-82%), median WBC was 2.1x10 9/L (0.6-26.4), and median platelets were 26x10 9/L (4-667). Pts had received a median of 1 (1-6) treatments prior to AML transformation. Prior diagnoses were: Chronic Myelomonocytic Leukemia (CMML), MDS, and MDS/MPN in 3 (25%), 14 (70%), and 1 (5%) respectively; all had received HMA, 11 (55%) additionally had received Ven, and 5 (25%) had stem cell transplantation (SCT) prior to enrolling. All pts had unfavorable cytogenetics by ELN 2022. The most frequent mutations were: TET2, TP53, RUNX1 and SRSF2 in 9 (45%), 7 (35%), 6 (30%) and 6 (30%) pts respectively. 18 pts were evaluable for e-selectin; median plasmatic concentration was 26.2ng/mL (15.3-131 ng/mL).
Most common AEs were grade(gr) ≥3 neutropenic fever (13 pts, 65%; including 2 gr 5 events), gr 3 bleeding (10%) and gr 2 thrombosis (5%). There were no dose-limiting toxicities observed on dose levels -1 or 1. The chosen dose level was 1 as described above. 2/3 pts who received dose level -1 died during the study follow-up due to sepsis in the first 4-week after induction. Median time to 0.5x10 9/L neutrophil and 50x10 9/L platelets recovery was 30 (17-52) and 38 (20-69) days, respectively.
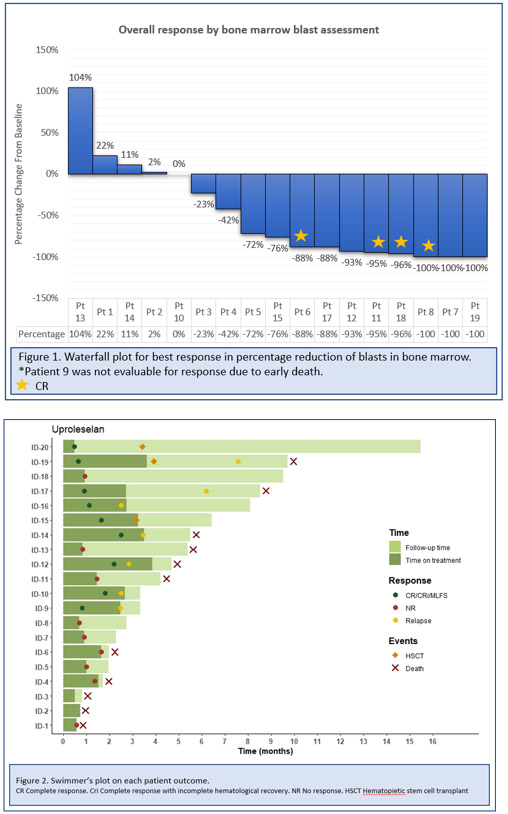
The response rates were 2 (11%) CRi, 1 (6%) CRp, and 4 (22%) MLFS. There was a reduction in BM blasts in 13 pts (72%) (Figure 1). The ORR was 50%(5/10) ( p=0.60) and 20% (1/5) ( p=0.06) among pts who had prior Ven exposure or prior SCT, respectively. The median cycles received was 1.5 (1-4), median cycles at which the best response was achieved was 1 (1-2). 12 pts were taken off protocol due to progression, 3 for death, 3 for allogeneic SCT and 1 continued in remission. The pt who achieved negative MRD-negative by flow cytometry underwent SCT and is still alive. The median follow-up is 8.1 months. Median OS and EFS were 5.3 and 1.4 months respectively; 4-month RFS (CRi, CRp and MLFS) was 30% (Fig 2). In the univariate analysis by Cox regression for plasmatic E-selectin concentration the HR for OS was 1.016 (95% CI 1.002-1.031).
Ts-AML, progressing from previously treated antecedent myeloid disorders, is associated with low rates of remission and poor OS. Uproleselan combined with Cladribine + LDAC was well-tolerated with minimal therapy-related AEs - allowing a safe approach to marrow blast reduction and disease control in preparation for a potential allogeneic SCT. We are currently determining the relationship of plasmatic e-selectin concentration and response to treatment.
Disclosures
Chien:AbbVie: Consultancy; Rigel Pharmaceuticals: Consultancy. DiNardo:Notable Labs: Honoraria; Novartis: Honoraria; ImmuniOnc: Honoraria; Servier: Honoraria; Fogham: Honoraria; BMS: Honoraria; Takeda: Honoraria; AbbVie/Genentech: Honoraria; Astellas: Honoraria; Schrödinger: Consultancy. Short:Amgen: Honoraria; Astellas: Research Funding; AstraZeneca: Consultancy; Stemline therapeutics: Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy; Pfizer: Consultancy. Maiti:Celgene: Research Funding; Lin BioScience: Research Funding. Montalban-Bravo:Takeda: Research Funding; Rigel: Research Funding. Ravandi:Syros: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Biomea fusion: Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding. Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding. Kadia:GenFleet Therapeutics: Research Funding; Sanofi-Aventis: Consultancy; Genentech: Consultancy, Research Funding; Janssen Research and Development: Research Funding; Pfizer: Consultancy, Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; SELLAS Life Sciences Group: Research Funding; Delta-Fly Pharma, Inc.: Research Funding; Amgen, Inc.: Research Funding; Astellas Pharma Global Development: Research Funding; Regeneron Pharmaceuticals: Research Funding; Glycomimetics: Research Funding; Cyclacel: Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Novartis: Consultancy; Cure: Speakers Bureau; Cellenkos Inc.: Research Funding; Ascentage Pharma Group: Research Funding; AstraZeneca: Research Funding; Celgene: Research Funding; Liberum: Consultancy; Iterion: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Genzyme: Honoraria; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Agios: Consultancy; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal